
The chiral altyl aryl backbone of the N-N axis is of interest to chemists because it is widely present in natural products, bioactive molecules, and chiral ligands (Figure 1a). However, compared with the widely studied C-C and C-N axis chiral compounds, the use of asymmetric catalysis to achieve high enantioselective synthesis of N-N axis chiral compounds started late, and has only been reported since 2021 (Chem 2021, 7, 2743; J. Am. Chem. Soc. 2021, 143, 15005; Figure 1b). On the other hand, although the C-H bond functional grouping reaction has been widely used in the asymmetric synthesis of axial chiral compounds, there are still few research efforts to obtain N-N axial chiral compounds using this strategy (Angew. Chem., Int. Ed. 2023, 62, e202218871)。
Based on the research interest of our research group in constructing novel axial chiral compounds by using asymmetric C-H bond functional clustering reactions, the team of You Shuli, a researcher at the Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, developed the asymmetric C-H bond alkylation reaction between iridium and acrylates catalyzed by the asymmetric aryl substrate and acrylate, and successfully realized the catalytic asymmetric synthesis of N-N axis chiral lientyl compounds (Figure 1C).
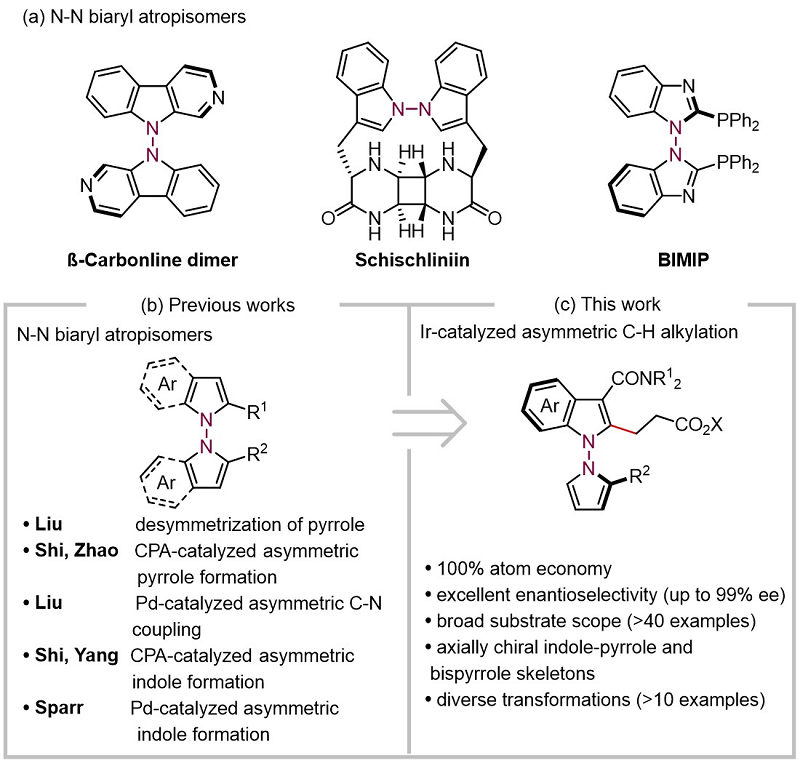
Figure 1. Catalytic asymmetric construction of N-N axis chiral liaryl compounds
Under optimal conditions, the authors investigated the universality of N-N axialized aryl substrates with acrylate substrates (Figure 2). The results showed that the reaction had good to excellent yield and enantioselectivity control (up to 98% yield, 99% EE) for both indole-pyrrole-aryl substrates and bispyrrole aryl substrates, and the acrylate substrates also had good compatibility to obtain target alkylation products with high enantioselectivity.
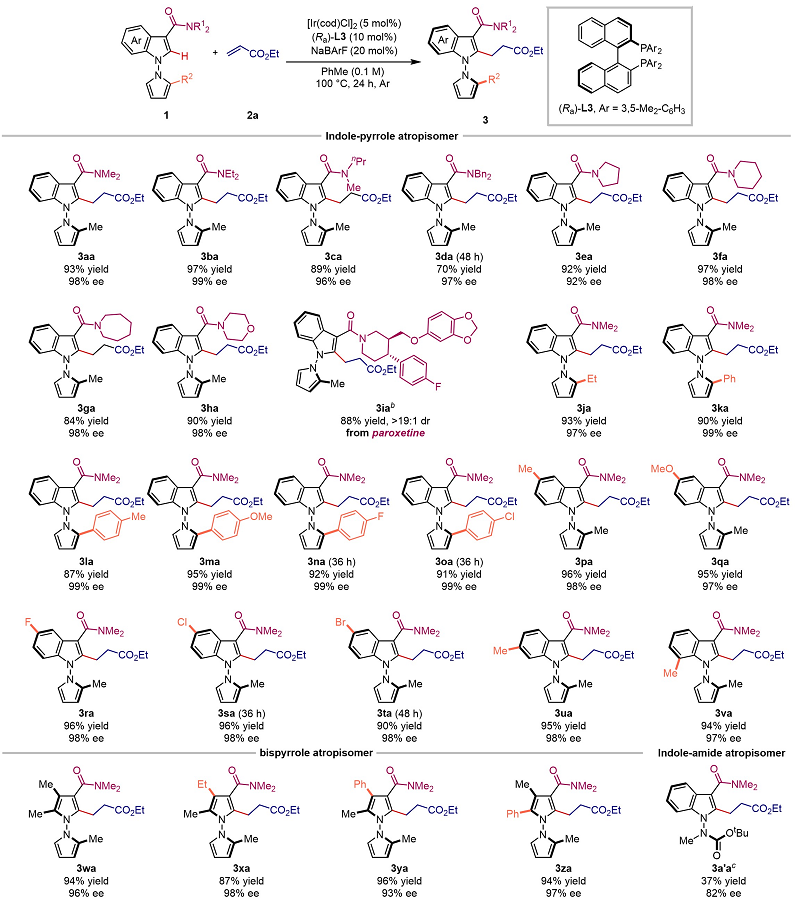
Figure 2. Universal study of N-N-linked aryl compounds
The reaction can be scaled up in grams, and the racemic barrier test of the product shows that these compounds have good axial chiral stability. Subsequently, the authors carried out transformation experiments on the products, and the products could be smoothly transformed such as bromination and lactamination, which provided the possibility for rapid acquisition of N-N axis chiral joint aryl compounds with diverse structures (Figure 3).
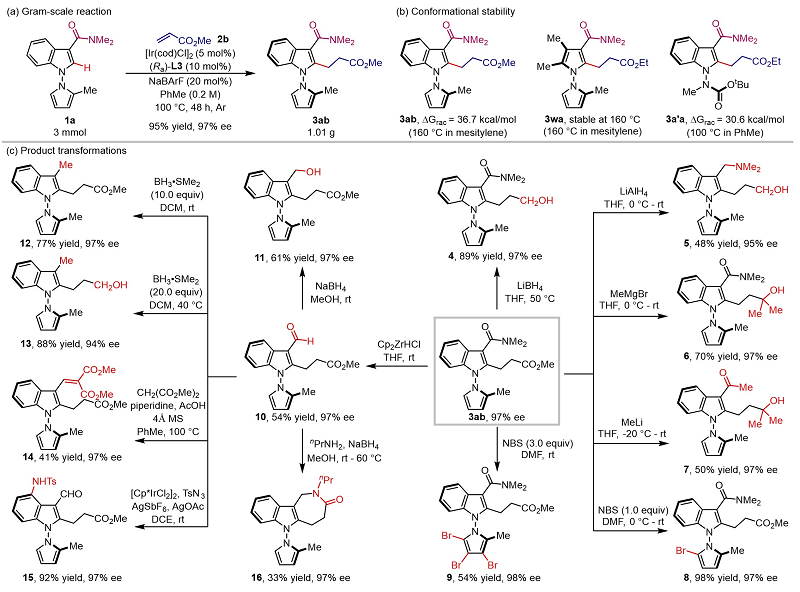
Figure 3. Racemic barrier testing and product transformation
The authors demonstrated through deuterated experiments that the reaction was initiated by the activation of the C-H bond of the N-N axialized aryl substrate by the catalyst, and the subsequent reaction was eliminated by migration insertion and reduction of acrylates to obtain the target product (Figure 4).
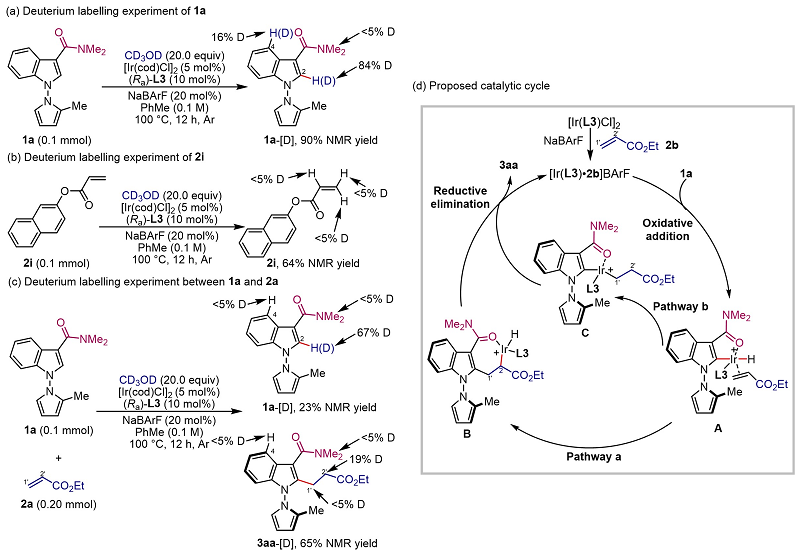
Figure 4. Mechanism experiments and possible catalytic cycling.
brief summary
In this work, the team of researcher You Shuli successfully constructed a class of indole-pyrrole and bispyrrole N-N axis chiral artyl compounds by using the iridium-catalyzed asymmetric C-H bond alkylation strategy, which provided a new method for the asymmetric synthesis of N-N axis chiral compounds. The diverse transformation of the product significantly increases the potential application value of the proposed method. The results were recently published in Angew. Chem., Int. Ed Previously, PhD student Yin Siyong is the first author of this paper, and researcher You Shuli is the corresponding author of this paper.
Name: Eric Yuan
Mobile:86-18427358861
Tel:86-18427358861
Whatsapp:+86 18427358861
Email:yihekeji@chembuying.com
Add: